Search results
Search for "epoxide-ring opening" in Full Text gives 37 result(s) in Beilstein Journal of Organic Chemistry.
Total synthesis of grayanane natural products
- Nicolas Fay,
- Rémi Blieck,
- Cyrille Kouklovsky and
- Aurélien de la Torre
Beilstein J. Org. Chem. 2022, 18, 1707–1719, doi:10.3762/bjoc.18.181
- -disubstituted olefin and reductive epoxide ring-opening giving triol 18. After oxidation of the primary and the secondary alcohols with Dess–Martin periodinane, the remaining tertiary alcohol was protected as a MOM ether and the silyl ether protecting group was removed. The obtained intermediate 19 was then a
- bromide coupling. While both Ding and Luo propose a 1,2-shift relying on epoxide ring-opening as key step, each group proposes a different disconnection. For Ding, the rearrangement forms bond C13–C16 through a Ti(III)-mediated reductive epoxide opening/Dowd–Beckwith rearrangement cascade. This allows to

Graphical Abstract

Figure 1: General structure of grayanane natural products.

Scheme 1: Grayanane biosynthesis.

Scheme 2: Matsumoto’s relay approach.

Scheme 3: Shirahama’s total synthesis of (–)-grayanotoxin III.

Scheme 4: Newhouse’s syntheses of fragments 25 and 29.

Scheme 5: Newhouse’s total synthesis of principinol D.

Scheme 6: Ding’s total synthesis of rhodomolleins XX and XXII.

Scheme 7: First key step of Luo’s strategy.

Scheme 8: Luo’s total synthesis of grayanotoxin III.

Scheme 9: Synthesis of principinol E and rhodomollein XX.

Scheme 10: William’s synthetic effort towards pierisformaside C.

Scheme 11: Hong’s synthetic effort towards rhodojaponin III.

Scheme 12: Recent strategies for grayanane synthesis.
Site-selective reactions mediated by molecular containers
- Rui Wang and
- Yang Yu
Beilstein J. Org. Chem. 2022, 18, 309–324, doi:10.3762/bjoc.18.35
- epoxide guest formed a 2:1 complex, and the internal reactive site of the epoxide was protected by the cyclodextrin host. Therefore, only the terminal site was attacked by the incoming hydride leading to epoxide-ring opening and formation of 1-phenyl-2-propanol (17). Utilizing the similar molecular

Graphical Abstract

Figure 1: Site-selective Diels–Alder reaction of anthracene and phthalimide mediated by aqueous organopalladi...

Figure 2: Site-selective Diels–Alder and [2 + 2]-photoaddition reactions between naphthalene and phthalimide ...

Figure 3: Cage host A-mediated selective 1,4-radical addition of o-quinone 10.

Figure 4: Cyclodextrin-mediated site-selective reductions.

Figure 5: Selective reduction of an α,ω-diazide compound mediated by water-soluble cavitand D.

Figure 6: Selective radical reduction of α,ω-dihalides mediated by water-soluble cavitands E and F.

Figure 7: Site-selective hydrogenation of polyenols mediated by supramolecular encapsulated rhodium catalyst.

Figure 8: Site-selective oxidation of steroids using cyclodextrin as the anchoring template.

Figure 9: Site-selective oxidations of linear diterpenoids with the help of cage host A.

Figure 10: Site-selective monoepoxidation of α,ω-dienes mediated by the water-soluble cavitand host E.

Figure 11: Site-selective ring-opening reaction of epoxides mediated by cavitand I with an inwardly directed c...

Figure 12: Site-selective nucleophilic substitution reaction of allylic chlorides mediated by cage host J.

Figure 13: Site-selective monohydrolysis of α,ω-difunctional compounds using deep water-soluble cavitands.
Efficient and regioselective synthesis of dihydroxy-substituted 2-aminocyclooctane-1-carboxylic acid and its bicyclic derivatives
- İlknur Polat,
- Selçuk Eşsiz,
- Uğur Bozkaya and
- Emine Salamci
Beilstein J. Org. Chem. 2022, 18, 77–85, doi:10.3762/bjoc.18.7
- the epoxide-ring-opening reaction of 7 with sodium azide, to introduce an extra amino group in position 4 on the cyclooctane skeleton. For this, epoxide 7 was treated with NaN3 in the presence of NH4Cl/DMF and this formed lactone 13 as the sole product in 80% yield (Scheme 4). The epoxide 7 was

Graphical Abstract

Figure 1: Examples of natural products containing β-amino acids.

Scheme 1: Synthesis of cyclic β-amino acid 6.

Scheme 2: Epoxidation of Boc-protected amino ester 4 and hydrolysis of epoxide 7 with HCl(g)–MeOH.

Scheme 3: Reaction of epoxide 7 with NaHSO4 in methylene chloride/MeOH.

Figure 2: The X-ray crystal structure of 10.

Scheme 4: Synthesis of cyclic β-amino acid derivative 8.

Scheme 5: Suggested mechanism for the reaction of epoxide 7 with NaHSO4.

Figure 3: Solvent-corrected relative free energy profile at 298.15 K for the reaction mechanism of 14 shown i...

Figure 4: Solvent-corrected relative free energy profile at 298.15 K for the reaction mechanism of 17 shown i...

Figure 5: The optimized geometries of the conformers 7a and 7b with selected interatomic distances at the B3L...
Highly stereocontrolled total synthesis of racemic codonopsinol B through isoxazolidine-4,5-diol vinylation
- Lukáš Ďurina,
- Anna Ďurinová,
- František Trejtnar,
- Ľuboš Janotka,
- Lucia Messingerová,
- Jana Doháňošová,
- Ján Moncol and
- Róbert Fischer
Beilstein J. Org. Chem. 2021, 17, 2781–2786, doi:10.3762/bjoc.17.188
- be prepared by substrate-directed epoxidation. A subsequent SN2 intramolecular epoxide ring-opening cyclization could provide an N-Cbz-protected pyrrolidine derivative with a hydroxymethyl group at C-5 and with trans configuration relative to the hydroxy group at C-4. Finally, (±)-codonopsinol B (1
- terminal alkenes bearing both an allylic and homoallylic-type hydroxy group, yielding a sole threo isomer, are rare [36]. The cyclization of 5 through an epoxide ring-opening reaction with boron trifluoride etherate in dichloromethane at 0 °C yielded pyrrolidine derivative 12 in 69% isolated yield [37

Graphical Abstract

Figure 1: (−)-Codonopsinol B (1) and its N-nor-methyl analogue 2; known inhibition activities against α-gluco...

Scheme 1: Synthetic approach towards (±)-codonopsinol B (1) and its N-nor-methyl analogue 2.

Scheme 2: Synthesis of isoxazolidine-4,5-diol (±)-3. Reagents and conditions: (a) ᴅʟ-proline, CHCl3, rt, 48 h...

Scheme 3: Synthesis of final pyrrolidines (±)-1 and (±)-2. Reagents and conditions: (a) vinyl-MgBr, CeCl3, TH...

Figure 2: Molecular structure of N-Cbz-protected pyrrolidine 12 confirmed by single-crystal X-ray crystallogr...
Double-headed nucleosides: Synthesis and applications
- Vineet Verma,
- Jyotirmoy Maity,
- Vipin K. Maikhuri,
- Ritika Sharma,
- Himal K. Ganguly and
- Ashok K. Prasad
Beilstein J. Org. Chem. 2021, 17, 1392–1439, doi:10.3762/bjoc.17.98
- ′-ketonucloside 1 with trimethylsulfoxonium iodide in DMSO afforded the spironucleoside 2, which in turn was converted to the TIPDS-protected 2′-(pyrimidin-1-yl)methyl-/2′-(purin-9-yl)methylarabinofuranosyluracil derivatives 3a–f by nucleophilic epoxide ring opening with thymine, N-benzoyladenine, 6-O-allyl-N
- ]. Nielsen and co-workers [42] additionally synthesized 2′-(N-benzoylcytosin-1-yl)methylarabinofuranosyl-N-benzoylcytosine (7) from uridine using a similar methodology. Thus, the nucleophilic epoxide ring opening in spironucleoside 2 with uracil in DMF in a N1-regioselective manner afforded the TIPDS

Graphical Abstract

Figure 1: Double-headed nucleosides. B1 and B2 = nucleobases or heterocyclic/carbocyclic moieties; L = linker....

Scheme 1: Synthesis of 2′-(pyrimidin-1-yl)methyl- or 2′-(purin-9-yl)methyl-substituted double-headed nucleosi...

Scheme 2: Synthesis of double-headed nucleoside 7 having two cytosine moieties.

Scheme 3: Synthesis of double-headed nucleoside 2′-deoxy-2′-C-(2-(thymine-1-yl)ethyl)-uridine (11).

Scheme 4: Double-headed nucleosides 14 and 15 obtained by click reaction.

Scheme 5: Synthesis of the double-headed nucleoside 19.

Scheme 6: Synthesis of the double-headed nucleosides 24 and 25.

Scheme 7: Synthesis of double-headed nucleosides 28 and 29.

Scheme 8: Synthesis of double-headed nucleoside 33.

Scheme 9: Synthesis of double-headed nucleoside 37.

Scheme 10: Synthesis of the double-headed nucleoside 1-(5′-O-(4,4′-dimethoxytrityl)-2′-C-((4-(pyren-1-yl)-1,2,...

Scheme 11: Synthesis of triazole-containing double-headed ribonucleosides 46a–c and 50a–e.

Scheme 12: Synthesis of double-headed nucleosides 54a–g.

Scheme 13: Synthesis of double-headed nucleosides 59 and 60.

Scheme 14: Synthesis of the double-headed nucleosides 63 and 64.

Scheme 15: Synthesis of double-headed nucleosides 66a–c.

Scheme 16: Synthesis of benzoxazole-containing double-headed nucleosides 69 and 71 from 5′-amino-5′-deoxynucle...

Scheme 17: Synthesis of 4′-C-((N6-benzoyladenin-9-yl)methyl)thymidine (75) and 4′-C-((thymin-1-yl)methyl)thymi...

Scheme 18: Synthesis of double-headed nucleosides 5′-(adenine-9-yl)-5′-deoxythymidine (79) and 5′-(adenine-9-y...

Scheme 19: Synthesis of double-headed nucleosides 85–87 via reversed nucleosides methodology.

Scheme 20: Double-headed nucleosides 91 and 92 derived from ω-terminal-acetylenic sugar derivatives 90a,b.

Scheme 21: Synthesis of double-headed nucleosides 96a–g.

Scheme 22: Synthesis of double-headed nucleosides 100 and 103.

Scheme 23: Double-headed nucleosides 104 and 105 with a triazole motif.

Scheme 24: Synthesis of the double-headed nucleosides 107 and 108.

Scheme 25: Synthesis of double-headed nucleoside 110 with additional nucleobase in 5′-(S)-C-position joined th...

Scheme 26: Synthesis of double-headed nucleosides 111–113 with additional nucleobases in the 5′-(S)-C-position...

Scheme 27: Synthesis of double-headed nucleoside 114 by click reaction.

Scheme 28: Synthesis of double-headed nucleosides 118 with an additional nucleobase at the 5′-(S)-C-position.

Scheme 29: Synthesis of bicyclic double-headed nucleoside 122.

Scheme 30: Synthesis of double-headed nucleosides 125a–c derived from 2′-amino-LNA.

Scheme 31: Double-headed nucleoside 127 obtained by click reaction.

Scheme 32: Synthesis of double-headed nucleoside 130.

Scheme 33: Double-headed nucleosides 132a–d and 134a–d synthesized by Sonogashira cross coupling reaction.

Scheme 34: Synthesis of double-headed nucleosides 137 and 138 via Suzuki coupling.

Scheme 35: Synthesis of double-headed nucleosides 140 and 141 via Sonogashira cross coupling reaction.

Scheme 36: Synthesis of double-headed nucleoside 143.

Scheme 37: Synthesis of the double-headed nucleoside 146.

Scheme 38: Synthesis of 5-C-alkynyl-functionalized double-headed nucleosides 151a–d.

Scheme 39: Synthesis of 5-C-triazolyl-functionalized double-headed nucleosides 154a, b.

Scheme 40: Synthesis of double-headed nucleosides 157a–c.

Scheme 41: Synthesis of double-headed nucleoside 159, phosphoramidite 160 and the corresponding nucleotide mon...

Scheme 42: Synthesis of double-headed nucleoside 163, phosphoramidite 164 and the corresponding nucleotide mon...

Scheme 43: Synthesis of double-headed nucleoside 167, phosphoramidite 168, and the corresponding nucleotide mo...

Scheme 44: Synthesis of double-headed nucleoside 171, phosphoramidite 172, and the corresponding nucleotide mo...

Scheme 45: Synthesis of double-headed nucleoside 175, phosphoramidite 176, and the corresponding nucleotide mo...

Scheme 46: Synthesis of double-headed nucleoside 178.

Scheme 47: Synthesis of the double-headed nucleosides 181 and 183.

Scheme 48: Alternative synthesis of the double-headed nucleoside 183.

Scheme 49: Synthesis of double-headed nucleoside 188 through thermal [2 + 3] sydnone–alkyne cycloaddition reac...

Scheme 50: Synthesis of the double-headed nucleosides 190 and 191.

Scheme 51: Synthesis of 1-((5S)-2,3,4-tri-O-acetyl-5-(2,6-dichloropurin-9-yl)-β-ᴅ-xylopyranosyl)uracil (195).

Scheme 52: Synthesis of hexopyranosyl double-headed pyrimidine homonucleosides 200a–c.

Figure 2: 3′-C-Ethynyl-β-ᴅ-allopyranonucleoside derivatives 201a–f.

Scheme 53: Synthesis of 3′-C-(1,4-disubstituted-1,2,3-triazolyl)-double-headed pyranonucleosides 203–207.

Scheme 54: Synthesis of 3′-C-(1,4-disubstituted-1,2,3-triazolyl)-double-headed pyranonucleosides 208 and 209.

Scheme 55: Synthesis of 3′-C-(1,4-disubstituted-1,2,3-triazolyl)-double-headed pyranonucleoside 210.

Scheme 56: Synthesis of double-headed acyclic nucleosides (2S,3R)-1,4-bis(thymine-1-yl)butane-2,3-diol (213a) ...

Scheme 57: Synthesis of double-headed acyclic nucleosides (2R,3S)-1,4-bis(thymine-1-yl)butane-2,3-diol (213c) ...

Scheme 58: Synthesis of double-headed acetylated 1,3,4-oxadiazino[6,5-b]indolium-substituted C-nucleosides 218b...

Scheme 59: Synthesis of double-headed acyclic nucleoside 222.

Scheme 60: Synthesis of functionalized 1,2-bis(1,2,4-triazol-3-yl)ethane-1,2-diols 223a–f.

Scheme 61: Synthesis of acyclic double-headed 1,2,4-triazino[5,6-b]indole C-nucleosides 226–231.

Scheme 62: Synthesis of double-headed 1,3,4-thiadiazoline, 1,3,4-oxadiazoline, and 1,2,4-triazoline acyclo C-n...

Scheme 63: Synthesis of double-headed acyclo C-nucleosides 240–242.

Scheme 64: Synthesis of double-headed acyclo C-nucleoside 246.

Scheme 65: Synthesis of acyclo double-headed nucleoside 250.

Scheme 66: Synthesis of acyclo double-headed nucleoside 253.

Scheme 67: Synthesis of acyclo double-headed nucleosides 259a–d.

Scheme 68: Synthesis of acyclo double-headed nucleoside 261.
Beyond ribose and phosphate: Selected nucleic acid modifications for structure–function investigations and therapeutic applications
- Christopher Liczner,
- Kieran Duke,
- Gabrielle Juneau,
- Martin Egli and
- Christopher J. Wilds
Beilstein J. Org. Chem. 2021, 17, 908–931, doi:10.3762/bjoc.17.76

- product is then reacted with unprotected thymine which, in the presence of stoichiometric amounts of sodium hydride, results in the epoxide ring opening and the formation of the glycol backbone. The pre-amidite is then phosphitylated yielding the desired GNA-T amidite (Scheme 3). Recently, this simple

Graphical Abstract

Figure 1: Structures of the chemically modified oligonucleotides (A) N3' → P5' phosphoramidate linkage, (B) a...

Scheme 1: Synthesis of a N3' → P5' phosphoramidate linkage by solid-phase synthesis. (a) dichloroacetic acid;...

Figure 2: Crystal structures of (A) N3' → P5' phosphoramidate DNA (PDB ID 363D) [71] and (B) amide (AM1) RNA in c...

Scheme 2: Synthesis of a phosphorodithioate linkage by solid-phase synthesis. (a) detritylation; (b) tetrazol...

Figure 3: Close-up view of a key interaction between the PS2-modified antithrombin RNA aptamer and thrombin i...

Scheme 3: Synthesis of the (S)-GNA thymine phosphoramidite from (S)-glycidyl 4,4'-dimethoxytrityl ether. (a) ...

Figure 4: Surface models of the crystal structures of RNA dodecamers with single (A) (S)-GNA-T (PDB ID 5V1L) [54]...

Figure 5: Structures of 2'-O-alkyl modifications. (A) 2'-O-methoxy RNA (2'-OMe RNA), (B) 2'-O-(2-methoxyethyl...

Scheme 4: Synthesis of the 2'-OMe uridine from 3',5'-O-(tetraisopropyldisiloxane-1,3-diyl)uridine. (a) Benzoy...

Scheme 5: Synthesis of the 2'-O-MOE uridine from uridine. (a) (PhO)2CO, NaHCO3, DMA, 100 °C; (b) Al(OCH2CH2OCH...

Figure 6: Structure of 2'-O-(2-methoxyethyl)-RNA (MOE-RNA). (A) View into the minor groove of an A-form DNA d...

Figure 7: Structures of locked nucleic acids (LNA)/bridged nucleic acids (BNA) modifications. (A) LNA/BNA, (B...

Scheme 6: Synthesis of the uridine LNA phosphoramidite. (a) i) NaH, BnBr, DMF, ii) acetic anhydride, pyridine...

Scheme 7: Synthesis of the 2'-fluoroarabinothymidine. (a) 30% HBr in acetic acid; (b) 2,4-bis-O-(trimethylsil...

Figure 8: Sugar puckers of arabinose (ANA) and arabinofluoro (FANA) nucleic acids compared with the puckers o...

Figure 9: Structures of C4'-modified nucleic acids. (A) 4'-methoxy, (B) 4'-(2-methoxyethoxy), (C) 2',4'-diflu...

Scheme 8: Synthesis of the 4'-F-rU phosphoramidite. (a) AgF, I2, dichloromethane, tetrahydrofuran; (b) NH3, m...

Scheme 9: Synthesis of the thymine FHNA phosphoramidite. (a) thymine, 1,8-diazabicyclo[5.4.0]undec-7-ene, ace...

Scheme 10: Synthesis of the thymine Ara-FHNA phosphoramidite. (a) i) trifluoromethanesulfonic anhydride, pyrid...

Figure 10: Crystal structures of (A) FHNA and (B) Ara-FHNA in modified A-form DNA decamers (PDB IDs 3Q61 and 3...
Microwave-assisted multicomponent reactions in heterocyclic chemistry and mechanistic aspects
- Shivani Gulati,
- Stephy Elza John and
- Nagula Shankaraiah
Beilstein J. Org. Chem. 2021, 17, 819–865, doi:10.3762/bjoc.17.71


Graphical Abstract

Figure 1: Marketed drugs with acridine moiety.

Scheme 1: Synthesis of 4-arylacridinediones.

Scheme 2: Proposed mechanism for acridinedione synthesis.

Scheme 3: Synthesis of tetrahydrodibenzoacridinones.

Scheme 4: Synthesis of naphthoacridines.

Scheme 5: Plausible mechanism for naphthoacridines.

Figure 2: Benzoazepines based potent molecules.

Scheme 6: Synthesis of azepinone.

Scheme 7: Proposed mechanism for azepinone formation.

Scheme 8: Synthesis of benzoazulenen-1-one derivatives.

Scheme 9: Proposed mechanism for benzoazulene-1-one synthesis.

Figure 3: Indole-containing pharmacologically active molecules.

Scheme 10: Synthesis of functionalized indoles.

Scheme 11: Plausible mechanism for the synthesis of functionalized indoles.

Scheme 12: Synthesis of spirooxindoles.

Scheme 13: Synthesis of substituted spirooxindoles.

Scheme 14: Plausible mechanism for the synthesis of substituted spirooxindoles.

Scheme 15: Synthesis of pyrrolidinyl spirooxindoles.

Scheme 16: Proposed mechanism for pyrrolidinyl spirooxindoles.

Figure 4: Pyran-containing biologically active molecules.

Scheme 17: Synthesis of functionalized benzopyrans.

Scheme 18: Plausible mechanism for synthesis of benzopyran.

Scheme 19: Synthesis of indoline-spiro-fused pyran derivatives.

Scheme 20: Proposed mechanism for indoline-spiro-fused pyran.

Scheme 21: Synthesis of substituted naphthopyrans.

Figure 5: Marketed drugs with pyrrole ring.

Scheme 22: Synthesis of tetra-substituted pyrroles.

Scheme 23: Mechanism for silica-supported PPA-SiO2-catalyzed pyrrole synthesis.

Scheme 24: Synthesis of pyrrolo[1,10]-phenanthrolines.

Scheme 25: Proposed mechanism for pyrrolo[1,10]-phenanthrolines.

Figure 6: Marketed drugs and molecules containing pyrimidine and pyrimidinones skeletons.

Scheme 26: MWA-MCR pyrimidinone synthesis.

Scheme 27: Two proposed mechanisms for pyrimidinone synthesis.

Scheme 28: MWA multicomponent synthesis of dihydropyrimidinones.

Scheme 29: Proposed mechanism for dihydropyrimidinones.

Figure 7: Biologically active fused pyrimidines.

Scheme 30: MWA- MCR for the synthesis of pyrrolo[2,3-d]pyrimidines.

Scheme 31: Proposed mechanism for pyrrolo[2,3-d]pyrimidines.

Scheme 32: Synthesis of substituted pyrrolo[2,3-d]pyrimidine-2,4-diones.

Scheme 33: Probable pathway for pyrrolo[2,3-d]pyrimidine-2,4-diones.

Scheme 34: Synthesis of pyridopyrimidines.

Scheme 35: Plausible mechanism for the synthesis of pyridopyrimidines.

Scheme 36: Synthesis of dihydropyridopyrimidine and dihydropyrazolopyridine.

Scheme 37: Proposed mechanism for the formation of dihydropyridopyrimidine.

Scheme 38: Synthesis of thiopyrano[4,3-d]pyrimidines.

Scheme 39: Plausible mechanism for the synthesis of thiopyrano[4,3-d]pyrimidines.

Scheme 40: Synthesis of decorated imidazopyrimidines.

Scheme 41: Proposed mechanism for imidazopyrimidine synthesis.

Figure 8: Pharmacologically active molecules containing purine bases.

Scheme 42: Synthesis of aza-adenines.

Scheme 43: Synthesis of 5-aza-7-deazapurines.

Scheme 44: Proposed mechanism for deazapurines synthesis.

Figure 9: Biologically active molecules containing pyridine moiety.

Scheme 45: Synthesis of steroidal pyridines.

Scheme 46: Proposed mechanism for steroidal pyridine.

Scheme 47: Synthesis of N-alkylated 2-pyridones.

Scheme 48: Two possible mechanisms for pyridone synthesis.

Scheme 49: Synthesis of pyridone derivatives.

Scheme 50: Postulated mechanism for synthesis of pyridone.

Figure 10: Biologically active fused pyridines.

Scheme 51: Benzimidazole-imidazo[1,2-a]pyridines synthesis.

Scheme 52: Mechanism for the synthesis of benzimidazole-imidazo[1,2-a]pyridines.

Scheme 53: Synthesis of pyrazolo[3,4-b]pyridine-5-spirocycloalkanedione derivatives.

Scheme 54: Proposed mechanism for spiro-pyridines.

Scheme 55: Functionalized macrocyclane-fused pyrazolo[3,4-b]pyridine derivatives.

Scheme 56: Mechanism postulated for macrocyclane-fused pyrazolo[3,4-b]pyridine.

Scheme 57: Generation of pyrazolo[3,4-b]pyridines.

Scheme 58: Proposed mechanism for the synthesis of pyrazolo[3,4-b]pyridines.

Scheme 59: Proposed mechanism for the synthesis of azepinoindole.

Figure 11: Pharmaceutically important molecules with quinoline moiety.

Scheme 60: Povarov-mediated quinoline synthesis.

Scheme 61: Proposed mechanism for Povarov reaction.

Scheme 62: Synthesis of pyrazoloquinoline.

Scheme 63: Plausible mechanism for pyrazoloquinoline synthesis.

Figure 12: Quinazolinones as pharmacologically significant scaffolds.

Scheme 64: Four-component reaction for dihydroquinazolinone.

Scheme 65: Proposed mechanism for dihydroquinazolinones.

Scheme 66: Synthesis purine quinazolinone and PI3K-δ inhibitor.

Scheme 67: Synthesis of fused benzothiazolo/benzoimidazoloquinazolinones.

Scheme 68: Proposed mechanism for fused benzothiazolo/benzoimidazoloquinazolinones.

Scheme 69: On-water reaction for synthesis of thiazoloquinazolinone.

Scheme 70: Proposed mechanism for the thiazoloquinazolinone synthesis.

Scheme 71: β-Cyclodextrin-mediated synthesis of indoloquinazolinediones.

Scheme 72: Proposed mechanism for synthesis of indoloquinazolinediones.

Figure 13: Triazoles-containing marketted drugs and pharmacologically active molecules.

Scheme 73: Cu(I) DAPTA-catalyzed 1,2,3-triazole formation.

Scheme 74: Mechanism for Cu(I) DAPTA-catalyzed triazole formation.

Scheme 75: Synthesis of β-hydroxy-1,2,3-triazole.

Scheme 76: Proposed mechanism for synthesis of β-hydroxy-1,2,3-triazoles.

Scheme 77: Synthesis of bis-1,2,4-triazoles.

Scheme 78: Proposed mechanism for bis-1,2,4-triazoles synthesis.

Figure 14: Thiazole containing drugs.

Scheme 79: Synthesis of a substituted thiazole ring.

Scheme 80: Synthesis of pyrazolothiazoles.

Figure 15: Chromene containing drugs.

Scheme 81: Magnetic nanocatalyst-mediated aminochromene synthesis.

Scheme 82: Proposed mechanism for the synthesis of chromenes.
α,γ-Dioxygenated amides via tandem Brook rearrangement/radical oxygenation reactions and their application to syntheses of γ-lactams
- Mikhail K. Klychnikov,
- Radek Pohl,
- Ivana Císařová and
- Ullrich Jahn
Beilstein J. Org. Chem. 2021, 17, 688–704, doi:10.3762/bjoc.17.58

- cyclizations to lactams of type 10 based on the persistent radical effect (PRE) are unknown and may provide a simple access to 3,4-disubstituted γ-lactams. We report here that tandem nucleophilic epoxide ring-opening/Brook rearrangement/radical oxygenation reactions are indeed very effective for the synthesis
- of the stereospecific epoxide ring-opening in 80% yield (not shown, see Supporting Information File 1 for details). N-Cyclopent-2-enyl and N-cyclohex-2-enylamides 8l,m provided the oxygenated products 9o,p in 68% and 63% yields, respectively, as 2:2:1:1 mixture of diastereomers (Scheme 2). Thus, the

Graphical Abstract

Figure 1: Selected alkaloids containing the pyrrolidone motif.

Scheme 1: A) Classical γ-lactam synthesis by atom transfer radical cyclizations; B) previously developed tand...

Figure 2: X-ray crystal structure of the major (2R,4S)-alkoxyamine hydrochloride derived from 9j. Displacemen...

Scheme 2: Formation of the α-(aminoxy)amides 9o,p.

Figure 3: X-ray crystal structure of the minor cis-diastereomers of the keto lactam 13j (left) and the hydrox...

Scheme 3: Thermal radical cyclization reactions of amides 9l–p bearing cyclic units. Conditions: a) t-BuOH, 1...

Scheme 4: Epimerization of spirolactams 12m,n.

Scheme 5: The Dess–Martin oxidation of lactams 12l–o. Conditions: a) DMP (1.3 equiv), t-BuOH (10 mol %), CH2Cl...

Scheme 6: Selected transformations of the lactams trans-12b and 12o.

Scheme 7: Diastereoselectivity for the formation of α-(aminoxy)amides 9i–k.

Scheme 8: Rationalization of the diastereoselectivity for the formation of the α-(aminoxy)amide 9l.

Scheme 9: Rationalization of the thermal radical cyclization diastereoselectivity of alkoxyamines 9a–k. (S)-C...

Scheme 10: The stereochemical course for the formation of products 12m,n by thermal radical cyclization of alk...

Scheme 11: Formation of bicycles 12o,p.
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
- Esra Demir,
- Ozlem Sari,
- Yasin Çetinkaya,
- Ufuk Atmaca,
- Safiye Sağ Erdem and
- Murat Çelik
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
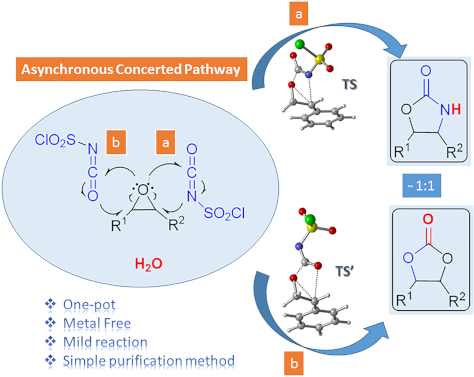
- ′ (Figure 2). Therefore, attack by N4 of CSI on the C2 of epoxide is found to be energetically the most favored approach. The epoxide ring opening and formation of the O–C(=O) bond are almost completed before the C–N bond is formed. The changes in bond lengths along the intrinsic reaction coordinate (IRC

Graphical Abstract

Scheme 1: Oxazolidinone (1), five-membered cyclic carbonate (2) and some important compounds containing an ox...

Scheme 2: Proposed mechanisms by Keshava Murthy and Dhar [41] and De Meijere and co-workers [42].

Figure 1: Possible pathways for the formation of oxazolidinone intermediates 10 and 11. Optimized transition ...

Figure 2: Potential energy profile related to the formation of oxazolidinone intermediates 10 and 11 at the P...

Figure 3: IRC calculated for the formation of (a) 10 and (b) 11 at M06-2X/6-31+G(d,p) level. I-1, I-15, I-35, ...

Figure 4: Optimized geometries for the stationary points for the formation of 10 at PCM(DCM)/M06-2X/6-31+G(d,...

Scheme 3: Proposed mechanisms for the formation of oxazolidinone 9f.

Figure 5: Potential energy profiles for paths 1a (blue), 1b (red), 2 (green) and relative Gibbs free energies...

Figure 6: Optimized geometries for the stationary points of path 1b at PCM(DCM)/M06-2X/6-31+G(d,p)//M06-2X/6-...

Scheme 4: Proposed mechanism for the formation of five-membered cyclic carbonate 8f.

Figure 7: Potential energy profile and relative Gibbs free energies (kcal/mol) in DCM related to the formatio...

Figure 8: Optimized geometries for the stationary points of step 1 for the formation of 16 at PCM(DCM)/M06-2X...

Figure 9: Optimized geometries for the stationary points of step 2 for the formation of 17 at PCM(DCM)/M06-2X...

Figure 10: Optimized geometries for the stationary points of step 3 for the formation of PC8 at PCM(DCM)/M06-2...
The charge-assisted hydrogen-bonded organic framework (CAHOF) self-assembled from the conjugated acid of tetrakis(4-aminophenyl)methane and 2,6-naphthalenedisulfonate as a new class of recyclable Brønsted acid catalysts
- Svetlana A. Kuznetsova,
- Alexander S. Gak,
- Yulia V. Nelyubina,
- Vladimir A. Larionov,
- Han Li,
- Michael North,
- Vladimir P. Zhereb,
- Alexander F. Smol'yakov,
- Artem O. Dmitrienko,
- Michael G. Medvedev,
- Igor S. Gerasimov,
- Ashot S. Saghyan and
- Yuri N. Belokon
Beilstein J. Org. Chem. 2020, 16, 1124–1134, doi:10.3762/bjoc.16.99
- , providing some dissolved F-1 as the real catalyst. In all cases, the catalyst could easily be recovered and recycled. Keywords: Brønsted acid catalyst; charge-assisted hydrogen-bonded framework; Diels–Alder; epoxide ring opening; heterogeneous catalyst; Introduction Tremendous successes in homogeneous
- “breathing”. The material served as a new type of Brønsted acid catalyst in a series of reactions, including epoxide ring opening reactions and a Diels–Alder reaction. A second role for NDS was that one of its sulfonate oxygen atoms could form hydrogen bonds with water whilst leaving the other two oxygen

Graphical Abstract

Scheme 1: The synthesis of F-1.

Figure 1: View of the crystal structure of F-1 (F-1a phase), with representation of atoms by thermal ellipsoi...

Figure 2: View of the crystal structure of F-1 (F-1a’ phase), with representation of the atoms via thermal el...

Figure 3: SEM image of F-1.

Figure 4: SEM image of F-1 with an F-1a phase.

Figure 5: TGA-DSC analysis of a sample of F-1. The TGA plot is shown in green, the DSC curve is shown in blue...

Scheme 2: Uncrystallized F-1 or F-1 with an F-1a phase promoted the two- and three-phase reactions of styrene...

Scheme 3: CAHOF F-1-promoted reactions of cyclohexene oxide (5) with alcohols and water.

Scheme 4: F-1-promoted Diels–Alder reaction.
Synthesis of acylglycerol derivatives by mechanochemistry
- Karen J. Ardila-Fierro,
- Andrij Pich,
- Marc Spehr,
- José G. Hernández and
- Carsten Bolm
Beilstein J. Org. Chem. 2019, 15, 811–817, doi:10.3762/bjoc.15.78

- by epoxide ring-opening and esterification reactions with fatty acids 3 (Scheme 1). If successful, developing a multistep approach to prepare DAGs would contribute to the expansion of synthetic mechanochemical methodologies in ball mills [28][29][30][31], which are often limited to single-step
- hands, a selective epoxide ring-opening reaction with fatty acids 3 leading to the formation of the corresponding sn-1,3-protected monoacylglycerols (MAGs 4) was attempted (Scheme 3). Initially, commonly used solution-based protocols were tested in the ball mill [33]. For example, 2 was reacted with
- -opening reaction of 2 with stearic acid (3a) was evaluated (Scheme 3a). Specifically, we focused on the use of Jacobsen cobalt(II)-salen complex (S,S)-cat (Scheme 3b), since similar salen complexes had originally been reported to facilitate epoxide ring-opening reactions with carboxylic acids as

Graphical Abstract

Figure 1: Biologically relevant molecules made, used or derivatized by mechanochemistry.

Figure 2: Isomeric diacyl-sn-glycerols (DAGs).

Scheme 1: Synthetic route to access protected DAGs; PG = protecting group.

Scheme 2: Protection of glycidol (1) with TBDMSCl in the ball mill. MM = mixer mill, PBM = planetary ball mil...

Scheme 3: Cobalt-catalyzed epoxide ring-opening in the ball mill.

Scheme 4: Mechanosynthesis of DAGs 5.

Scheme 5: Conjugation of DAG 5a with 7-hydroxycoumarin (9).

Figure 3: UV−vis spectra of DAG 6a (dotted line) and conjugated DAGs 10a and 10a’ as a mixture (10a/10a’ 72:2...
Synthesis of nonracemic hydroxyglutamic acids
- Dorota G. Piotrowska,
- Iwona E. Głowacka,
- Andrzej E. Wróblewski and
- Liwia Lubowiecka
Beilstein J. Org. Chem. 2019, 15, 236–255, doi:10.3762/bjoc.15.22

- acid Cleavage of the 5-membered ring in the protected epoxide 88 obtained from (S)-pyroglutamic acid [93][94][95] gave the methyl ester 89 which, when adsorbed on silica gel, smoothly underwent stereospecific epoxide ring opening to give the oxazolidinone 90 (Scheme 23) [96]. Before installation of the

Graphical Abstract

Figure 1: Structure of L-glutamic acid.

Figure 2: 3-Hydroxy- (2), 4-hydroxy- (3) and 3,4-dihydroxyglutamic acids (4).

Figure 3: Enantiomers of 3-hydroxyglutamic acid (2).

Scheme 1: Synthesis of (2S,3R)-2 from (R)-Garner's aldehyde. Reagents and conditions: a) MeOCH=CH–CH(OTMS)=CH2...

Scheme 2: Synthesis of (2S,3R)-2 and (2S,3S)-2 from (R)-Garner’s aldehyde. Reagents and conditions: a) H2C=CH...

Scheme 3: Two-carbon homologation of the protected L-serine. Reagents and conditions: a) Fmoc-succinimide, Na2...

Scheme 4: Synthesis of di-tert-butyl ester of (2R,3S)-2 from L-serine. Reagents and conditions: a) PhSO2Cl, K2...

Scheme 5: Synthesis of (2R,3S)-2 from O-benzyl-L-serine. Reagents and conditions: a) (CF3CH2O)2P(O)CH2COOMe, ...

Scheme 6: Synthesis of (2S,3R)-2 employing a one-pot cis-olefination–conjugate addition sequence. Reagents an...

Scheme 7: Synthesis of the orthogonally protected (2S,3R)-2 from a chiral aziridine. Reagents and conditions:...

Scheme 8: Synthesis of N-Boc-protected (2S,3R)-2 from D-phenylglycine. Reagents and conditions: a) BnMgCl, et...

Scheme 9: Synthesis of (2S,3R)-2 employing ketopinic acid as chiral auxiliary. Reagents and conditions: a) Br2...

Scheme 10: Synthesis of dimethyl ester of (2S,3R)-2 employing (1S)-2-exo-methoxyethoxyapocamphane-1-carboxylic...

Scheme 11: Synthesis of N-Boc-protected dimethyl ester of (2S,3R)-2 from (S)-N-(1-phenylethyl)thioacetamide. R...

Scheme 12: Synthesis of N-Boc-protected dimethyl ester of (2S,3R)-2 via Sharpless epoxidation. Reagents and co...

Scheme 13: Synthesis of (2S,3S)-2 from the imide 51. Reagents and conditions: a) NaBH4, MeOH/CH2Cl2; b) Ac2O, ...

Scheme 14: Synthesis of (2R,3S)-2 and (2S,3S)-2 from the acetolactam 55 (PMB = p-methoxybenzyl). Reagents and ...

Scheme 15: Synthesis of (2S,3R)-2 from D-glucose. Reagents and conditions: a) NaClO2, 30% H2O2, NaH2PO4, MeCN;...

Figure 4: Enantiomers of 3-hydroxyglutamic acid (3).

Scheme 16: Synthesis of (4S)-4-hydroxy-L-glutamic acid [(2S,4S)-3] by electrophilic hydroxylation. Reagents an...

Scheme 17: Synthesis of all stereoisomers of 4-hydroxyglutamic acid (3). Reagents and conditions: a) Br2, PBr5...

Scheme 18: Synthesis of the orthogonally protected 4-hydroxyglutamic acid (2S,4S)-73. Reagents and conditions:...

Scheme 19: Synthesis of (2S,4R)-4-acetyloxyglutamic acid as a component of a dipeptide. Reagents and condition...

Scheme 20: Synthesis of N-Boc-protected dimethyl esters of (2S,4R)- and (2S,4S)-3 from (2S,4R)-4-hydroxyprolin...

Scheme 21: Synthesis of orthogonally protected (2S,4S)-3 from (2S,4R)-4-hydroxyproline. Reagents and condition...

Scheme 22: Synthesis of the protected (4R)-4-hydroxy-L-pyroglutamic acid (2S,4R)-87 by electrophilic hydroxyla...

Figure 5: Enantiomers of 3,4-dihydroxy-L-glutamic acid (4).

Scheme 23: Synthesis of (2S,3S,4R)-4 from the epoxypyrrolidinone 88. Reagents and conditions: a) MeOH, THF, KC...

Scheme 24: Synthesis of (2S,3R,4R)-4 from the orthoester 92. Reagents and conditions: a) OsO4, NMO, acetone/wa...

Scheme 25: Synthesis of (2S,3S,4S)-4 from the aziridinolactone 95. Reagents and conditions: a) BnOH, BF3·OEt2,...

Scheme 26: Synthesis of (2S,3S,4R)-4 and (2R,3S,4R)-4 from cyclic imides 106. Reagents and conditions: a) NaBH4...

Scheme 27: Synthesis of (2R,3R,4R)-4 and (2S,3R,4R)-4 from the cyclic meso-imide 110. Reagents and conditions:...

Scheme 28: Synthesis of (2S,3S,4S)-4 from the protected serinal (R)-23. Reagents and conditions: a) Ph3P=CHCOO...

Scheme 29: Synthesis of (2S,3S,4S)-4 from O-benzyl-N-Boc-D-serine. Reagents and conditions: a) ClCOOiBu, TEA, ...

Scheme 30: Synthesis of (2S,3S,4R)-127 by enantioselective conjugate addition and asymmetric dihydroxylation. ...

Figure 6: Structures of selected compounds containing hydroxyglutamic motives (in blue).
Functionalization of graphene: does the organic chemistry matter?
- Artur Kasprzak,
- Agnieszka Zuchowska and
- Magdalena Poplawska
Beilstein J. Org. Chem. 2018, 14, 2018–2026, doi:10.3762/bjoc.14.177
- , (c) reaction with DMAP, (d and e) desired reaction pathway (ester or amide bond formation), (d and f) reaction of the activated carboxyl group with water molecules. Mechanism of the epoxide ring opening reaction with the GO/RGO. Generation of the free amine (nucleophile) from the corresponding amine

Graphical Abstract

Figure 1: Partial structure [7,8] of the (a) graphene oxide (GO) and (b) reduced graphene oxide (RGO).

Figure 2: Mechanism of the amidation/esterification-type reactions with the GO/RGO using carbodiimide and N-h...

Figure 3: Mechanism of the Steglich esterification with the GO/RGO: (a) acid–base reaction of the carboxyl gr...

Figure 4: Mechanism of the epoxide ring opening reaction with the GO/RGO.

Figure 5: Generation of the free amine (nucleophile) from the corresponding amine hydrohalide using an acid–b...

Figure 6: Mechanism of amidation/esterification-type reactions with the GO/RGO using 1,1’-carbonyldiimidazole...

Figure 7: Mechanism of the covalent functionalization of graphene-family material applying diazonium salts ch...
β-Hydroxy sulfides and their syntheses
- Mokgethwa B. Marakalala,
- Edwin M. Mmutlane and
- Henok H. Kinfe
Beilstein J. Org. Chem. 2018, 14, 1668–1692, doi:10.3762/bjoc.14.143
- . Regioselective epoxide ring opening and 1,2-difunctionalization of alkenes are the commonly employed routes in the synthesis of such compounds. Both strategies are discussed below. 3.1 Synthesis of β-hydroxy sulfides via regioselective ring opening of epoxides The considerable ring strain present in epoxides
- . Besides the ability of the catalysts to promote the epoxide ring opening with sulfur nucleophiles at room temperature, the attractiveness of the promoter was its capability to effect opening of the epoxide ring with alcohols, selenols and amines to provide the corresponding β-hydroxy analogs. 3.1.1.2
- good yields of products and remarkable regioselectivity in most cases are obtained. Devan et al. reported a one-pot, multistep tetrathiomolybdate-assisted epoxide ring opening with masked thiolates and selenoates [60]. Treatment of epoxides with in situ-generated disulfides in the presence of

Graphical Abstract

Figure 1: Some sulfur-containing natural products.

Figure 2: Some natural products incorporating β-hydroxy sulfide moieties.

Figure 3: Some synthetic β-hydroxy sulfides of clinical value.

Scheme 1: Alumina-mediated synthesis of β-hydroxy sulfides, ethers, amines and selenides from epoxides.

Scheme 2: β-Hydroxy sulfide syntheses by ring opening of epoxides under different Lewis and Brønsted acid and...

Scheme 3: n-Bu3P-catalyzed thiolysis of epoxides and aziridines to provide the corresponding β-hydroxy and β-...

Scheme 4: Zinc(II) chloride-mediated thiolysis of epoxides.

Scheme 5: Thiolysis of epoxides and one-pot oxidation to β-hydroxy sulfoxides under microwave irradiation.

Scheme 6: Gallium triflate-catalyzed ring opening of epoxides and one-pot oxidation.

Scheme 7: Thiolysis of epoxides and one-pot oxidation to β-hydroxy sulfoxides using Ga(OTf)3 as a catalyst.

Scheme 8: Ring opening of epoxide using ionic liquids under solvent-free conditions.

Scheme 9: N-Bromosuccinimide-catalyzed ring opening of epoxides.

Scheme 10: LiNTf2-mediated epoxide opening by thiophenol.

Scheme 11: Asymmetric ring-opening of cyclohexene oxide with various thiols catalyzed by zinc L-tartrate.

Scheme 12: Catalytic asymmetric ring opening of symmetrical epoxides with t-BuSH catalyzed by (R)-GaLB (43) wi...

Scheme 13: Asymmetric ring opening of meso-epoxides by p-xylenedithiol catalyzed by a (S,S)-(salen)Cr complex.

Scheme 14: Desymmetrization of meso-epoxide with thiophenol derivatives.

Scheme 15: Enantioselective ring-opening reaction of meso-epoxides with ArSH catalyzed by a C2-symmetric chira...

Scheme 16: Enantioselective ring-opening reaction of stilbene oxides with ArSH catalyzed by a C2-symmetric chi...

Scheme 17: Asymmetric desymmetrization of meso-epoxides using BINOL-based Brønsted acid catalysts.

Scheme 18: Lithium-BINOL-phosphate-catalyzed desymmetrization of meso-epoxides with aromatic thiols.

Scheme 19: Ring-opening reactions of cyclohexene oxide with thiols by using CPs 1-Eu and 2-Tb.

Scheme 20: CBS-oxazaborolidine-catalyzed borane reduction of β-keto sulfides.

Scheme 21: Preparation of β-hydroxy sulfides via connectivity.

Scheme 22: Baker’s yeast-catalyzed reduction of sulfenylated β-ketoesters.

Scheme 23: Sodium-mediated ring opening of epoxides.

Scheme 24: Disulfide bond cleavage-epoxide opening assisted by tetrathiomolybdate.

Scheme 25: Proposed reaction mechanism of disulfide bond cleavage-epoxide opening assisted by tetrathiomolybda...

Scheme 26: Cyclodextrin-catalyzed difunctionalization of alkenes.

Scheme 27: Zinc-catalyzed synthesis of β-hydroxy sulfides from disulfides and alkenes.

Scheme 28: tert-Butyl hydroperoxide-catalyzed hydroxysulfurization of alkenes.

Scheme 29: Proposed mechanism of the radical hydroxysulfurization.

Scheme 30: Rongalite-mediated synthesis of β-hydroxy sulfides from styrenes and disulfides.

Scheme 31: Proposed mechanism of Rongalite-mediated synthesis of β-hydroxy sulfides from styrenes and disulfid...

Scheme 32: Copper(II)-catalyzed synthesis of β-hydroxy sulfides 15e,f from alkenes and basic disulfides.

Scheme 33: CuI-catalyzed acetoxysulfenylation of alkenes.

Scheme 34: CuI-catalyzed acetoxysulfenylation reaction mechanism.

Scheme 35: One-pot oxidative 1,2-acetoxysulfenylation of Baylis–Hillman products.

Scheme 36: Proposed mechanism for the oxidative 1,2-acetoxysulfination of Baylis–Hillman products.

Scheme 37: 1,2-Acetoxysulfenylation of alkenes using DIB/KI.

Scheme 38: Proposed reaction mechanism of the diacetoxyiodobenzene (DIB) and KI-mediated 1,2-acetoxysulfenylat...

Scheme 39: Catalytic asymmetric thiofunctionalization of unactivated alkenes.

Scheme 40: Proposed catalytic cycle for asymmetric sulfenofunctionalization.

Scheme 41: Synthesis of thiosugars using intramolecular thiol-ene reaction.

Scheme 42: Synthesis of leukotriene C-1 by Corey et al.: (a) N-(trifluoroacetyl)glutathione dimethyl ester (3 ...

Scheme 43: Synthesis of pteriatoxins with epoxide thiolysis to attain β-hydroxy sulfides. Reagents: (a) (1) K2...

Scheme 44: Synthesis of peptides containing a β-hydroxy sulfide moiety.

Scheme 45: Synthesis of diltiazem (12) using biocatalytic resolution of an epoxide followed by thiolysis.
Recent applications of chiral calixarenes in asymmetric catalysis
- Mustafa Durmaz,
- Erkan Halay and
- Selahattin Bozkurt
Beilstein J. Org. Chem. 2018, 14, 1389–1412, doi:10.3762/bjoc.14.117

- exhibited the best result. Aza-Diels–Alder and epoxide ring-opening reaction Manoury et al. have very recently reported facile synthesis of an enantiomerically pure inherently chiral calix[4]arene phosphonic acid (cR,pR)-121 from the readily available (cS)-enantiomer of calix[4]arene acetic acid 119 or its
- important transformations: phase-transfer catalysis, Henry reaction, Suzuki–Miyaura cross-coupling and Tsuji–Trost allylic substitution, hydrogenation, Michael addition, aldol and multicomponent Biginelli reactions, epoxidation, Meerwein–Ponndorf–Verley reduction, aza-Diels–Alder and epoxide ring-opening
- reaction in the following order: phase-transfer catalysis, Henry reaction, Suzuki–Miyaura cross-coupling and Tsuji–Trost allylic substitution, hydrogenation, Michael addition, aldol and multicomponent Biginelli reactions, epoxidation, Meerwein−Ponndorf−Verley reduction, aza-Diels−Alder and epoxide ring

Graphical Abstract

Figure 1: Inherently chiral calix[4]arene-based phase-transfer catalysts.

Scheme 1: Asymmetric alkylations of 3 catalyzed by (±)-1 and (±)-2 under phase-transfer conditions.

Scheme 2: Synthesis of chiral calix[4]arene-based phase-transfer catalyst 7 and structure of O’Donnell’s N-be...

Scheme 3: Asymmetric alkylation of glycine derivative 3 catalyzed by calixarene-based phase-transfer catalyst ...

Figure 2: Calix[4]arene-amides used as phase-transfer catalysts.

Scheme 4: Phase-transfer alkylation of 3 catalyzed by calixarene-triamide 12.

Scheme 5: Synthesis of inherently chiral calix[4]arenes 20a/20b substituted at the lower rim. Reaction condit...

Scheme 6: Asymmetric Henry reaction between 21 and 22 catalyzed by 20a/20b.

Figure 3: Proposed transition state model of asymmetric Henry reaction.

Scheme 7: Synthesis of enantiomerically pure phosphinoferrocenyl-substituted calixarene ligands 27–29.

Scheme 8: Asymmetric coupling reaction of aryl boronates and aryl halides in the presence of calixarene mono ...

Scheme 9: Asymmetric allylic alkylation in the presence of calix[4]arene ligand (S,S)-29.

Figure 4: Structure of inherently chiral oxazoline calix[4]arenes applied in the palladium-catalyzed Tsuji–Tr...

Scheme 10: Asymmetric Tsuji–Trost reaction in the presence of calix[4]arene ligands 36–39.

Figure 5: BINOL-derived calix[4]arene-diphosphite ligands.

Scheme 11: Asymmetric hydrogenation of 41a and 41b catalyzed by in situ-generated catalysts comprised of [Rh(C...

Figure 6: Inherently chiral calix[4]arene 43 containing a diarylmethanol structure.

Scheme 12: Asymmetric Michael addition reaction of 44 with 45 catalyzed by 43.

Figure 7: Calix[4]arene-based chiral primary amine–thiourea catalysts.

Scheme 13: Asymmetric Michael addition of 48 with 49 catalyzed by 47a and 47b.

Scheme 14: Enantioselective Michael addition of 51 to 52 catalyzed by calix[4]arene thioureas.

Scheme 15: Synthesis of calix[4]arene-based tertiary amine–thioureas 54–56.

Scheme 16: Asymmetric Michael addition of 34 and 57 to nitroalkenes 49 catalyzed by 54b.

Scheme 17: Synthesis of p-tert-butylcalix[4]arene bis-squaramide derivative 64.

Scheme 18: Asymmetric Michael addition catalyzed by 64.

Scheme 19: Synthesis of chiral p-tert-butylphenol analogue 68.

Figure 8: Novel prolinamide organocatalysts based on the calix[4]arene scaffold.

Scheme 20: Asymmetric aldol reactions of 72 with 70 and 71 catalyzed by 69b.

Scheme 21: Synthesis of p-tert-butylcalix[4]arene-based chiral organocatalysts 75 and 78 derived from L-prolin...

Scheme 22: Synthesis of upper rim-functionalized calix[4]arene-based L-proline derivative 83.

Scheme 23: Synthesis and proposed structure of Calix-Pro-MN (86).

Figure 9: Calix[4]arene-based L-proline catalysts containing ester, amide and acid units.

Scheme 24: Synthesis of calix[4]arene-based prolinamide 92.

Scheme 25: Calixarene-based catalysts for the aldol reaction of 21 with 70.

Scheme 26: Asymmetric aldol reactions of 72 with cyclic ketones catalyzed by calix[4]arene-based chiral organo...

Figure 10: A proposed structure for catalyst 92 in H2O.

Scheme 27: Synthetic route for organocatalyst 98.

Scheme 28: Asymmetric aldol reactions catalyzed by 99.

Figure 11: Proposed catalytic environment for catalyst 99 in the presence of water.

Scheme 29: Asymmetric aldol reactions between 94 and 72 catalyzed by 55a.

Scheme 30: Enantioselective Biginelli reactions catalyzed by 69f.

Scheme 31: Synthesis of calix[4]arene–(salen) complexes.

Scheme 32: Enantioselective epoxidation of 108 catalyzed by 107a/107b.

Scheme 33: Synthesis of inherently chiral calix[4]arene catalysts 111 and 112.

Scheme 34: Enantioselective MPV reduction.

Scheme 35: Synthesis of chiral calix[4]arene ligands 116a–c.

Scheme 36: Asymmetric MPV reduction with chiral calix[4]arene ligands.

Scheme 37: Chiral AlIII–calixarene complexes bearing distally positioned chiral substituents.

Scheme 38: Asymmetric MPV reduction in the presence of chiral calix[4]arene diphosphites.

Scheme 39: Synthesis of enantiomerically pure inherently chiral calix[4]arene phosphonic acid.

Scheme 40: Asymmetric aza-Diels–Alder reactions catalyzed by (cR,pR)-121.

Scheme 41: Asymmetric ring opening of epoxides catalyzed by (cR,pR)-121.
Homologated amino acids with three vicinal fluorines positioned along the backbone: development of a stereoselective synthesis
- Raju Cheerlavancha,
- Ahmed Ahmed,
- Yun Cheuk Leung,
- Aggie Lawer,
- Qing-Quan Liu,
- Marina Cagnes,
- Hee-Chan Jang,
- Xiang-Guo Hu and
- Luke Hunter
Beilstein J. Org. Chem. 2017, 13, 2316–2325, doi:10.3762/bjoc.13.228
- have previously reported a concise method for synthesising compounds that contain three vicinal C–F bonds [34]; their method commences with an epoxy alcohol, which undergoes three successive nucleophilic substitutions with fluoride (i.e., deoxyfluorination of the alcohol, epoxide ring opening with

Graphical Abstract

Figure 1: Examples of conformationally biased amino acids [1-10]. Compound 6 is a target of this work.

Scheme 1: The first synthetic approach.

Scheme 2: The second synthetic approach.

Scheme 3: The third synthetic approach.

Scheme 4: The fourth synthetic approach (partially reproduced from ref. [17]).

Figure 2: Selected J values and the inferred molecular conformations of 6a and 6b.
Studies directed toward the exploitation of vicinal diols in the synthesis of (+)-nebivolol intermediates
- Runjun Devi and
- Sajal Kumar Das
Beilstein J. Org. Chem. 2017, 13, 571–578, doi:10.3762/bjoc.13.56
- the possibility of obtaining 2 via intramolecular epoxide ring-opening of 7 (Scheme 1, method 2). Consequently, an alternative pathway involving the Mitsunobu inversion of 9 (obtained by intramolecular epoxide ring-opening of 8 which is the enantiomer of 6) has been followed to obtain 2 (Scheme 1
- reaction [34] to obtain the β-hydroxy-α-tosyloxy esters 24 and 25, respectively (Scheme 6). Panda and co-worker applied a three-step reaction sequence involving epoxidation/debenzylation/epoxide ring-opening to convert the β-hydroxy-α-tosyloxy ester into the corresponding 2-substituted chroman derivative
- formation. Further we speculated that compound 26, in the presence of a base, might undergo a simultaneous epoxidation–intramolecular epoxide-ring opening to produce 27 (Scheme 7) as the corresponding benzoxepin ring formation via intramolecular displacement of –OTs group by ArO− is unresponsive [23]. To

Graphical Abstract

Figure 1: The chroman-based antihypertensive drug nebivolol, its biologically active stereoisomers and late-s...

Scheme 1: Synthetic strategies toward late-stage intermediates of 1a.

Scheme 2: Attempted synthesis of (±)-2 via intramolecular SNAr reaction.

Scheme 3: Speculation on the synthesis of a 2-substituted chroman derivative based on Borhan’s approach.

Scheme 4: Synthesis of syn-2,3-dihydroxy esters 19 and 20.

Scheme 5: Attempted cyclization according to Borhan’s method.

Scheme 6: Synthesis of β-hydroxy-α-tosyloxy esters 24 and 25.

Scheme 7: Speculation of simultaneous epoxidation/epoxide-ring opening.

Scheme 8: Synthesis of chroman diols 2 and 29, respectively.

Scheme 9: Conversion of 32 into 3 via Mitsunobu inversion.

Scheme 10: Synthesis of chroman epoxide 5.
cis-Diastereoselective synthesis of chroman-fused tetralins as B-ring-modified analogues of brazilin
- Dimpee Gogoi,
- Runjun Devi,
- Pallab Pahari,
- Bipul Sarma and
- Sajal Kumar Das
Beilstein J. Org. Chem. 2016, 12, 2816–2822, doi:10.3762/bjoc.12.280
- literature reports on bioactivities of brazilin, haematoxylin and their analogues and our fruitful experience [22][23][25][26] in intramolecular epoxide ring-opening chemistry including IFCEA cyclization, we became interested in the possibility of extending the IFCEA cyclization protocol to the
- that the [6,5]-ring system in brazilin and related natural products 1–4 remains cis-fused, its corresponding [6,6]-ring system in 5 can have cis or trans stereochemistry at the ring junction. In our previous work, we observed a cis relationship (both in concerted or/and stepwise-epoxide ring opening
- ) between the 4-aryl group and H atom at the C-3 position of 4-arylchroman-3-ols (thus giving rise to trans-4-arylchroman-3-ols) [22][23]. But it was not obvious how the planned IFCEA cyclization onto the pre-existing 6-membered ring, in case of stepwise-epoxide ring opening, would influence the product

Graphical Abstract

Figure 1: Chroman-based tetracyclic natural products 1–4 of the brazilin family and our designed, B-ring-modi...

Scheme 1: Retrosynthetic analysis of the designed B-ring-modified analogues of brazilin.

Scheme 2: The synthetic challenge associated with the synthesis of 5 by IFCEA of 6 (above) and recent literat...

Figure 2: Assessment of the IFCEA cyclization on additional substrates (±)-6b–n leading to (±)-5b–n. Reaction...

Figure 3: ORTEP diagram of 5k.

Scheme 3: Stereoselective conversion of (±)-5k into (±)-14.
Synthesis of the C8’-epimeric thymine pyranosyl amino acid core of amipurimycin
- Pramod R. Markad,
- Navanath Kumbhar and
- Dilip D. Dhavale
Beilstein J. Org. Chem. 2016, 12, 1765–1771, doi:10.3762/bjoc.12.165
- oxocarbenium ion at C1 to which concomitant addition of a hydroxy group (present in the side chain at C3) will give the requisite pyranose ring skeleton. Intermediate B could be derived from the allyl alcohol C by using the Sharpless asymmetric epoxidation followed by regioselective epoxide ring opening with
- the ratio of 18:82 in 83% yield. With the understanding of SAE mnemonic, we assigned the absolute configuration in epoxide 8 as 7S,8S and in epoxide 9 as 7R,8R. Subsequently, major isomers of epoxy alcohols 8 and 9 were individually subjected to regioselective epoxide ring opening using trimethyl

Graphical Abstract

Figure 1: Antifungal antibiotic amipurimycin (1).

Scheme 1: Retrosynthesis of 2.

Scheme 2: Synthesis of 1,3-anhydrosugar 12 and 13.

Scheme 3: Formation of 2,7-dioxabicyclo[3.2.1]octane 12/13.

Figure 2: Conformational analysis of 13 and 14.

Figure 3: Geometrically optimized conformation of 12 and 13 respectively by DFT study.

Scheme 4: Glycosylation of 16.

Scheme 5: Glycosylation attempt by changing protections.

Scheme 6: Synthesis of nucleoside 2.
Efficient syntheses of climate relevant isoprene nitrates and (1R,5S)-(−)-myrtenol nitrate
- Sean P. Bew,
- Glyn D. Hiatt-Gipson,
- Graham P. Mills and
- Claire E. Reeves
Beilstein J. Org. Chem. 2016, 12, 1081–1095, doi:10.3762/bjoc.12.103
- ] (Scheme 2, path B); Cohen et al. reported the application of bismuth(III) nitrate for isoprene epoxide ring-opening/trapping with nitrate [19] (Scheme 2, path C). The 2010 report by Shepson et al. (path A) exploited chemistry originally described by Nichols et al. who, employing nitric acid as a

Graphical Abstract

Scheme 1: Simplified overview outlining how a small number of different IPNs are synthesised and are able to ...

Scheme 2: Protocols for the synthesis of O-nitrated alcohols using (±)-isoprene epoxide and 2° alcohols as st...

Scheme 3: Attempted synthesis of O-nitrate ester rac-19 and rac-20 synthesis.

Scheme 4: Olah et al. O-nitrated alcohol syntheses of 23–33 using N-nitro-2,4-6-trimethylpyridinium tetrafluo...

Scheme 5: O-nitration study using 22 and the alcohols 34–37.

Scheme 6: Silver nitrate mediated synthesis of 2-oxopropyl nitrate 43.

Scheme 7: Application of isoprene for the synthesis of precursors to IPNs and synthesis via ‘halide for nitra...

Scheme 8: Synthesis of (E)-3-methyl-4-chlorobut-2-en-1-ol ((E)-60) and (Z)-3-methyl-4-chlorobut-2-en-1-ol ((Z...

Scheme 9: Using NOESY interactions to establish the conformations of the C=C bonds within (E)-10 and (Z)-9.

Scheme 10: Synthesis of isoprene nitrates (E)-11 and (Z)-12 from ketone 63.

Scheme 11: Attempted synthesis of rac-8 from O-mesylate rac-71.

Scheme 12: Synthesis of O-nitrate 73 from O-mesylate 72.

Scheme 13: Attempted synthesis of 2° alcohol containing 1° nitrate ester rac-19 and the unexpected synthesis o...

Scheme 14: Synthesis of monoterpene derived (1R,5S)-(−)-myrtenol nitrate 86.
A carbohydrate approach for the formal total synthesis of (−)-aspergillide C
- Pabbaraja Srihari,
- Namballa Hari Krishna,
- Ydhyam Sridhar and
- Ahmed Kamal
Beilstein J. Org. Chem. 2014, 10, 3122–3126, doi:10.3762/bjoc.10.329
- on aspergillides, Achmatowicz adducts were utilized as the key source for the construction of the dihydropyran moiety and the side arm was synthesized using a Zipper rearrangement as a key reaction after an epoxide ring opening reaction of (R)-propylene oxide/(S)-propylene oxide with n-BuLi. In the

Graphical Abstract

Figure 1: Structures of aspergillides.

Scheme 1: Retrosynthetic analysis for (−)-aspergillide C.

Scheme 2: Synthesis of 11. Reaction conditions: (a) n-BuLi, THF/HMPA (5:1), −78 °C to rt, 12 h, 95%; (b) Li, t...

Figure 2: Key NOESY correlations observed in compound 11.

Scheme 3: Synthesis of 4 and formal total synthesis of (−)-aspergillide C (3). Reaction conditions: (a) [Cp*(...
Regio- and stereoselective synthesis of new diaminocyclopentanols
- Evgeni A. Larin,
- Valeri S. Kochubei and
- Yuri M. Atroshchenko
Beilstein J. Org. Chem. 2014, 10, 2513–2520, doi:10.3762/bjoc.10.262
- .10.262 Abstract The optimal conditions for regio- and stereoselective epoxide ring opening of N,N-disubstituted 1,2-epoxy-3-aminocyclopentanes by different nucleophilic reagents have been developed. The substituents on the nitrogen atom in the epoxide precursor and the orientation of the oxirane ring are
- -purin-6-amine (7d) were used as nucleophiles (Figure 1). Starting amines were selected based on the fact that these motifs are common structural features in drug molecules. Optimization of the epoxide ring opening reaction of 3a The opening of epoxides with nucleophiles in the presence of Lewis acid or
- base promoters is well documented [30][31][32][33][34]. We conducted a number of experiments to optimize the ring opening in 3a (Table 1). The initial catalytic epoxide ring-opening experiments of 3a in MeCN at 80 °C [35] were unsuccessful, since only starting material was recovered. A series of

Graphical Abstract

Scheme 1: Preparation of the starting materials.

Figure 1: Amine-based nucleophiles used in the epoxide ring opening reaction.

Scheme 2: Postulated mechanism for the formation of 14a,b.
An economical and safe procedure to synthesize 2-hydroxy-4-pentynoic acid: A precursor towards ‘clickable’ biodegradable polylactide
- Quanxuan Zhang,
- Hong Ren and
- Gregory L. Baker
Beilstein J. Org. Chem. 2014, 10, 1365–1371, doi:10.3762/bjoc.10.139
- sublimated under vacuum to give 24.1 g of acid 1 in 49.0% yield [17]. 1H NMR (500 MHz, CDCl3) δ 4.35 (t, J = 4.5 Hz, 1H), 2.68 (m, 2H), 2.08 (t, J = 2.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 177.5, 77.7, 71.9, 68.5, 24.4. Synthesis of 2-hydroxy-4-pentynoic acid (1). Synthesis of 1 via epoxide ring opening with

Graphical Abstract

Scheme 1: Synthesis of 2-hydroxy-4-pentynoic acid (1).

Scheme 2: Synthesis of 1 via epoxide ring opening with organoalane.

Scheme 3: Attempted synthetic routes for compound 1.

Scheme 4: Synthesis of 2-hydroxy-4-pentynoic acid (1) from diethyl 2-acetamidomalonate.
Molecular recognition of surface-immobilized carbohydrates by a synthetic lectin
- Melanie Rauschenberg,
- Eva-Corrina Fritz,
- Christian Schulz,
- Tobias Kaufmann and
- Bart Jan Ravoo
Beilstein J. Org. Chem. 2014, 10, 1354–1364, doi:10.3762/bjoc.10.138

- HisHis towards immobilized NANA in comparison with the glycosides of glucose (Glc), galactose (Gal) and mannose (Man) (Figure 1). In this study, we exploit the epoxide ring opening reaction of amine-tethered carbohydrates on epoxide-terminated SAMs [42] to print carbohydrate microarrays on silicon and
- epoxide ring opening reaction with amines is much faster at elevated temperatures, the surface of oxidized, patterned PDMS stamps was coated with 2-[methoxy(polyethyleneoxy)propyl]trimethylsilane (PEG silane) [51]. The PEG coated stamps provided optimal wetting of the stamp by the carbohydrate ink

Graphical Abstract

Figure 1: Molecular structures of carbohydrates (NANA, Glc, Gal, Man) immobilized on epoxide SAMs, NANA-bindi...

Figure 2: Schematic representation of the preparation of a simple carbohydrate microarray by μCP of amine-fun...

Figure 3: Optical microscopy images of water droplets selectively condensed in the areas where (A) the NANA i...

Figure 4: (A) AFM height image (zoom) of NANA ink in 10 μm stripes on an epoxide-terminated SAM; (B) Height p...

Figure 5: Fluorescence images of bifunctional carbohydrate microarrays incubated with FITC-HisHis. (A) NANA (...

Figure 6: Overlay of fluorescence images of bifunctional carbohydrate microarrays; (A) NANA (dots 10 × 5 μm) ...

Figure 7: Fluorescence images of a microarray consisting of NANA (dots 5 × 3 μm) and Man (background). (A) In...

Figure 8: Fluorescence images of a microarray of NANA (dots 5 × 3 μm) and Glc (background), first incubated w...
Synthesis of five- and six-membered cyclic organic peroxides: Key transformations into peroxide ring-retaining products
- Alexander O. Terent'ev,
- Dmitry A. Borisov,
- Vera A. Vil’ and
- Valery M. Dembitsky
Beilstein J. Org. Chem. 2014, 10, 34–114, doi:10.3762/bjoc.10.6

Graphical Abstract

Figure 1: Five and six-membered cyclic peroxides.

Figure 2: Artemisinin and semi-synthetic derivatives.

Scheme 1: Synthesis of 3-hydroxy-1,2-dioxolanes 3a–c.

Scheme 2: Synthesis of dioxolane 6.

Scheme 3: Photooxygenation of oxazolidines 7a–d with formation of spiro-fused oxazolidine-containing dioxolan...

Scheme 4: Oxidation of cyclopropanes 10a–e and 11a–e with preparation of 1,2-dioxolanes 12a–e.

Scheme 5: VO(acac)2-catalyzed oxidation of silylated bicycloalkanols 13a–c.

Scheme 6: Mn(II)-catalyzed oxidation of cyclopropanols 15a–g.

Scheme 7: Oxidation of aminocyclopropanes 20a–c.

Scheme 8: Synthesis of aminodioxolanes 24.

Figure 3: Trifluoromethyl-containing dioxolane 25.

Scheme 9: Synthesis of 1,2-dioxolanes 27a–e by the oxidation of cyclopropanes 26a–e.

Scheme 10: Photoinduced oxidation of methylenecyclopropanes 28.

Scheme 11: Irradiation-mediated oxidation.

Scheme 12: Application of diazene 34 for dioxolane synthesis.

Scheme 13: Mn(OAc)3-catalyzed cooxidation of arylacetylenes 37a–h and acetylacetone with atmospheric oxygen.

Scheme 14: Peroxidation of (2-vinylcyclopropyl)benzene (40).

Scheme 15: Peroxidation of 1,4-dienes 43a,b.

Scheme 16: Peroxidation of 1,5-dienes 46.

Scheme 17: Peroxidation of oxetanes 53a,b.

Scheme 18: Peroxidation of 1,6-diene 56.

Scheme 19: Synthesis of 3-alkoxy-1,2-dioxolanes 62a,b.

Scheme 20: Synthesis of spiro-bis(1,2-dioxolane) 66.

Scheme 21: Synthesis of dispiro-1,2-dioxolanes 68, 70, 71.

Scheme 22: Synthesis of spirohydroperoxydioxolanes 75a,b.

Scheme 23: Synthesis of spirohydroperoxydioxolane 77 and dihydroperoxydioxolane 79.

Scheme 24: Ozonolysis of azepino[4,5-b]indole 80.

Scheme 25: SnCl4-mediated fragmentation of ozonides 84a–l in the presence of allyltrimethylsilane.

Scheme 26: SnCl4-mediated fragmentation of bicyclic ozonide 84m in the presence of allyltrimethylsilane.

Scheme 27: MCl4-mediated fragmentation of alkoxyhydroperoxides 96 in the presence of allyltrimethylsilane.

Scheme 28: SnCl4-catalyzed reaction of monotriethylsilylperoxyacetal 108 with alkene 109.

Scheme 29: SnCl4-catalyzed reaction of triethylsilylperoxyacetals 111 with alkenes.

Scheme 30: Desilylation of tert-butyldimethylsilylperoxy ketones 131a,b followed by cyclization.

Scheme 31: Deprotection of peroxide 133 followed by cyclization.

Scheme 32: Asymmetric peroxidation of methyl vinyl ketones 137a–e.

Scheme 33: Et2NH-catalyzed intramolecular cyclization.

Scheme 34: Synthesis of oxodioxolanes 143a–j.

Scheme 35: Haloperoxidation accompanied by intramolecular ring closure.

Scheme 36: Oxidation of triterpenes 149a–d with Na2Cr2O7/N-hydroxysuccinimide.

Scheme 37: Curtius and Wolff rearrangements to form 1,2-dioxolane ring-retaining products.

Scheme 38: Oxidative desilylation of peroxide 124.

Scheme 39: Synthesis of dioxolane 158, a compound containing the aminoquinoline antimalarial pharmacophore.

Scheme 40: Diastereomers of plakinic acid A, 162a and 162b.

Scheme 41: Ozonolysis of alkenes.

Scheme 42: Cross-ozonolysis of alkenes 166 with carbonyl compounds.

Scheme 43: Ozonolysis of the bicyclic cyclohexenone 168.

Scheme 44: Cross-ozonolysis of enol ethers 172a,b with cyclohexanone.

Scheme 45: Griesbaum co-ozonolysis.

Scheme 46: Reactions of aryloxiranes 177a,b with oxygen.

Scheme 47: Intramolecular formation of 1,2,4-trioxolane 180.

Scheme 48: Formation of 1,2,4-trioxolane 180 by the reaction of 1,5-ketoacetal 181 with H2O2.

Scheme 49: 1,2,4-Trioxolane 186 with tetrazole fragment.

Scheme 50: 1,2,4-Trioxolane 188 with a pyridine fragment.

Scheme 51: 1,2,4-Trioxolane 189 with pyrimidine fragment.

Scheme 52: Synthesis of aminoquinoline-containing 1,2,4-trioxalane 191.

Scheme 53: Synthesis of arterolane.

Scheme 54: Oxidation of diarylheptadienes 197a–c with singlet oxygen.

Scheme 55: Synthesis of hexacyclinol peroxide 200.

Scheme 56: Oxidation of enone 201 and enenitrile 203 with singlet oxygen.

Scheme 57: Synthesis of 1,2-dioxanes 207 by oxidative coupling of carbonyl compounds 206 and alkenes 205.

Scheme 58: 1,2-Dioxanes 209 synthesis by co-oxidation of 1,5-dienes 208 and thiols.

Scheme 59: Synthesis of bicyclic 1,2-dioxanes 212 with aryl substituents.

Scheme 60: Isayama–Mukaiyama peroxysilylation of 1,5-dienes 213 followed by desilylation under acidic conditio...

Scheme 61: Synthesis of bicycle 218 with an 1,2-dioxane ring.

Scheme 62: Intramolecular cyclization with an oxirane-ring opening.

Scheme 63: Inramolecular cyclization with the oxetane-ring opening.

Scheme 64: Intramolecular cyclization with the attack on a keto group.

Scheme 65: Peroxidation of the carbonyl group in unsaturated ketones 228 followed by cyclization of hydroperox...

Scheme 66: CsOH and Et2NH-catalyzed cyclization.

Scheme 67: Preparation of peroxyplakoric acid methyl ethers A and D.

Scheme 68: Hg(OAc)2 in 1,2-dioxane synthesis.

Scheme 69: Reaction of 1,4-diketones 242 with hydrogen peroxide.

Scheme 70: Inramolecular cyclization with oxetane-ring opening.

Scheme 71: Inramolecular cyclization with MsO fragment substitution.

Scheme 72: Synthesis of 1,2-dioxane 255a, a structurally similar compound to natural peroxyplakoric acids.

Scheme 73: Synthesis of 1,2-dioxanes based on the intramolecular cyclization of hydroperoxides containing C=C ...

Scheme 74: Use of BCIH in the intramolecular cyclization.

Scheme 75: Palladium-catalyzed cyclization of δ-unsaturated hydroperoxides 271a–e.

Scheme 76: Intramolecular cyclization of unsaturated peroxyacetals 273a–d.

Scheme 77: Allyltrimethylsilane in the synthesis of 1,2-dioxanes 276a–d.

Scheme 78: Intramolecular cyclization using the electrophilic center of the peroxycarbenium ion 279.

Scheme 79: Synthesis of bicyclic 1,2-dioxanes.

Scheme 80: Preparation of 1,2-dioxane 286.

Scheme 81: Di(tert-butyl)peroxalate-initiated radical cyclization of unsaturated hydroperoxide 287.

Scheme 82: Oxidation of 1,4-betaines 291a–d.

Scheme 83: Synthesis of aminoquinoline-containing 1,2-dioxane 294.

Scheme 84: Synthesis of the sulfonyl-containing 1,2-dioxane.

Scheme 85: Synthesis of the amido-containing 1,2-dioxane 301.

Scheme 86: Reaction of singlet oxygen with the 1,3-diene system 302.

Scheme 87: Synthesis of (+)-premnalane А and 8-epi-premnalane A.

Scheme 88: Synthesis of the diazo group containing 1,2-dioxenes 309a–e.

Figure 4: Plakortolide Е.

Scheme 89: Synthesis of 6-epiplakortolide Е.

Scheme 90: Application of Bu3SnH for the preparation of tetrahydrofuran-containing bicyclic peroxides 318a,b.

Scheme 91: Application of Bu3SnH for the preparation of lactone-containing bicyclic peroxides 320a–f.

Scheme 92: Dihydroxylation of the double bond in the 1,2-dioxene ring 321 with OsO4.

Scheme 93: Epoxidation of 1,2-dioxenes 324.

Scheme 94: Cyclopropanation of the double bond in endoperoxides 327.

Scheme 95: Preparation of pyridazine-containing bicyclic endoperoxides 334a–c.

Scheme 96: Synthesis of 1,2,4-trioxanes 337 by the hydroperoxidation of unsaturated alcohols 335 with 1O2 and ...

Scheme 97: Synthesis of sulfur-containing 1,2,4-trioxanes 339.

Scheme 98: BF3·Et2O-catalyzed synthesis of the 1,2,4-trioxanes 342a–g.

Scheme 99: Photooxidation of enol ethers or vinyl sulfides 343.

Scheme 100: Synthesis of tricyclic peroxide 346.

Scheme 101: Reaction of endoperoxides 348a,b derived from cyclohexadienes 347a,b with 1,4-cyclohexanedione.

Scheme 102: [4 + 2]-Cycloaddition of singlet oxygen to 2Н-pyrans 350.

Scheme 103: Synthesis of 1,2,4-trioxanes 354 using peroxysilylation stage.

Scheme 104: Epoxide-ring opening in 355 with H2O2 followed by the condensation of hydroxy hydroperoxides 356 wi...

Scheme 105: Peroxidation of unsaturated ketones 358 with the H2O2/CF3COOH/H2SO4 system.

Scheme 106: Synthesis of 1,2,4-trioxanes 362 through Et2NH-catalyzed intramolecular cyclization.

Scheme 107: Reduction of the double bond in tricyclic peroxides 363.

Scheme 108: Horner–Wadsworth–Emmons reaction in the presence of peroxide group.

Scheme 109: Reduction of ester group by LiBH4 in the presence of 1,2,4-trioxane moiety.

Scheme 110: Reductive amination of keto-containing 1,2,4-trioxane 370.

Scheme 111: Reductive amination of keto-containing 1,2,4-trioxane and a Fe-containing moiety.

Scheme 112: Acid-catalyzed reactions of Н2О2 with ketones and aldehydes 374.

Scheme 113: Cyclocondensation of carbonyl compounds 376a–d using Me3SiOOSiMe3/CF3SO3SiMe3.

Scheme 114: Peroxidation of 4-methylcyclohexanone (378).

Scheme 115: Synthesis of symmetrical tetraoxanes 382a,b from aldehydes 381a,b.

Scheme 116: Synthesis of unsymmetrical tetraoxanes using of MeReO3.

Scheme 117: Synthesis of symmetrical tetraoxanes using of MeReO3.

Scheme 118: Synthesis of symmetrical tetraoxanes using of MeReO3.

Scheme 119: MeReO3 in the synthesis of symmetrical tetraoxanes with the use of aldehydes.

Scheme 120: Preparation of unsymmmetrical 1,2,4,5-tetraoxanes with high antimalarial activity.

Scheme 121: Re2O7-Catalyzed synthesis of tetraoxanes 398.

Scheme 122: H2SO4-Catalyzed synthesis of steroidal tetraoxanes 401.

Scheme 123: HBF4-Catalyzed condensation of bishydroperoxide 402 with 1,4-cyclohexanedione.

Scheme 124: BF3·Et2O-Catalyzed reaction of gem-bishydroperoxides 404 with enol ethers 405 and acetals 406.

Scheme 125: HBF4-Catalyzed cyclocondensation of bishydroperoxide 410 with ketones.

Scheme 126: Synthesis of symmetrical and unsymmetrical tetraoxanes 413 from benzaldehydes 412.

Scheme 127: Synthesis of bridged 1,2,4,5-tetraoxanes 415a–l from β-diketones 414a–l and H2O2.

Scheme 128: Dimerization of zwitterions 417.

Scheme 129: Ozonolysis of verbenone 419.

Scheme 130: Ozonolysis of O-methyl oxime 424.

Scheme 131: Peroxidation of 1,1,1-trifluorododecan-2-one 426 with oxone.

Scheme 132: Intramolecular cyclization of dialdehyde 428 with H2O2.

Scheme 133: Tetraoxanes 433–435 as by-products in peroxidation of ketals 430–432.

Scheme 134: Transformation of triperoxide 436 in diperoxide 437.

Scheme 135: Preparation and structural modifications of tetraoxanes.

Scheme 136: Structural modifications of steroidal tetraoxanes.

Scheme 137: Synthesis of 1,2,4,5-tetraoxane 454 containing the fluorescent moiety.

Scheme 138: Synthesis of tetraoxane 458 (RKA182).






